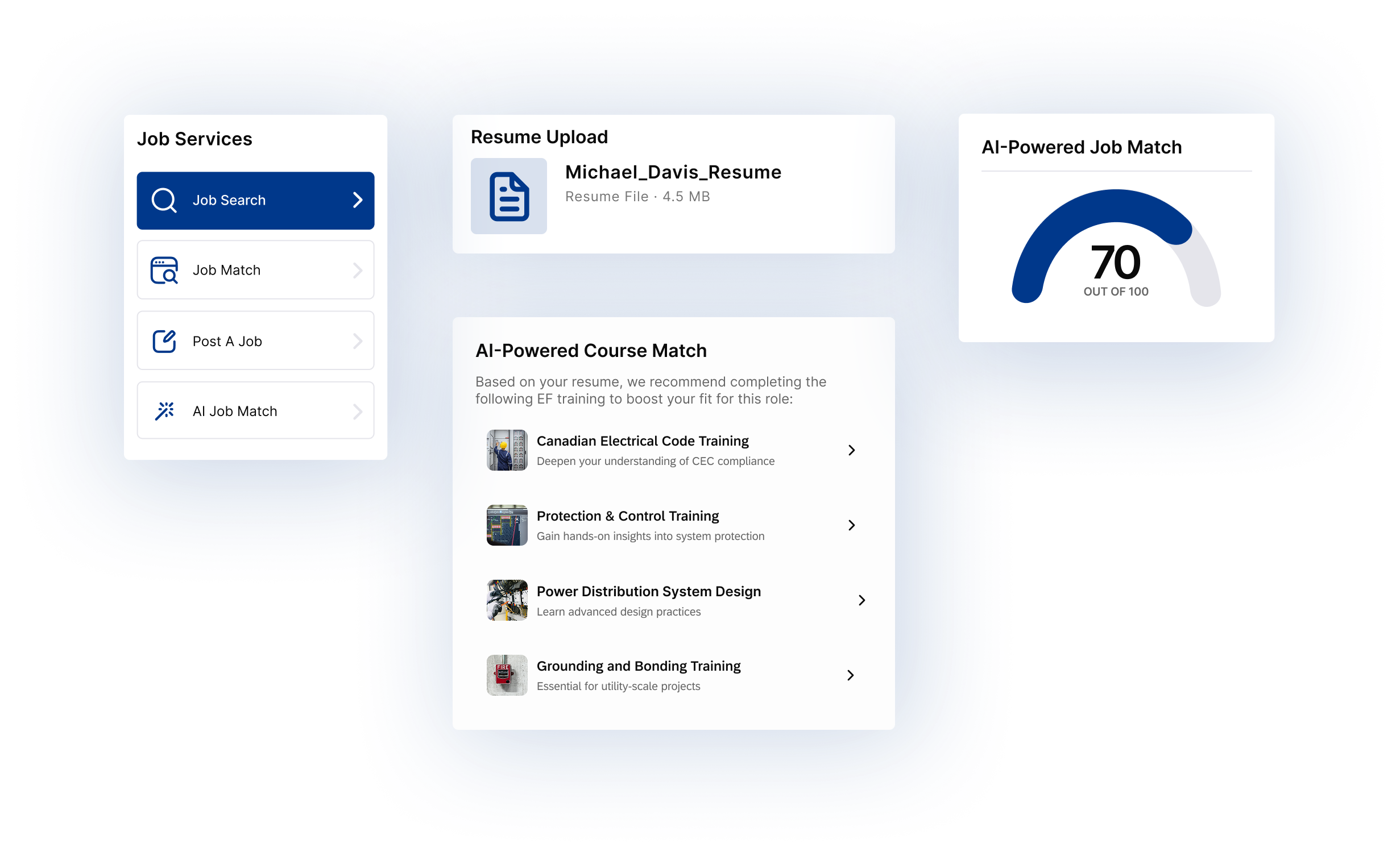
New fuel cell could help fix the renewable energy storage problem

NFPA 70e Training
Our customized live online or in‑person group training can be delivered to your staff at your location.

- Live Online
- 6 hours Instructor-led
- Group Training Available
Proton Conducting Fuel Cells enable reversible hydrogen energy storage, coupling electrolyzers and fuel cells with ceramic catalysts and proton-conducting membranes to convert wind and solar electricity into fuel and back to reliable grid power.
Key Points
Proton conducting fuel cells store renewable power as hydrogen and generate electricity using reversible catalysts.
✅ Reversible electrolysis and fuel-cell operation in one device
✅ Ceramic air electrodes hit up to 98% splitting efficiency
✅ Scalable path to low-cost grid energy storage with hydrogen
If we want a shot at transitioning to renewable energy, we’ll need one crucial thing: technologies that can convert electricity from wind, sun, and even electricity from raindrops into a chemical fuel for storage and vice versa. Commercial devices that do this exist, but most are costly and perform only half of the equation. Now, researchers have created lab-scale gadgets that do both jobs. If larger versions work as well, they would help make it possible—or at least more affordable—to run the world on renewables.
The market for such technologies has grown along with renewables: In 2007, solar and wind provided just 0.8% of all power in the United States; in 2017, that number was 8%, according to the U.S. Energy Information Administration. But the demand for electricity often doesn’t match the supply from solar and wind, a key reason why the U.S. grid isn't 100% renewable today. In sunny California, for example, solar panels regularly produce more power than needed in the middle of the day, but none at night, after most workers and students return home.
Some utilities are beginning to install massive banks of cheaper solar batteries in hopes of storing excess energy and evening out the balance sheet. But batteries are costly and store only enough energy to back up the grid for a few hours at most. Another option is to store the energy by converting it into hydrogen fuel. Devices called electrolyzers do this by using electricity—ideally from solar and wind power—to split water into oxygen and hydrogen gas, a carbon-free fuel. A second set of devices called fuel cells can then convert that hydrogen back to electricity to power cars, trucks, and buses, or to feed it to the grid.
But commercial electrolyzers and fuel cells use different catalysts to speed up the two reactions, meaning a single device can’t do both jobs. To get around this, researchers have been experimenting with a newer type of fuel cell, called a proton conducting fuel cell (PCFC), which can make fuel or convert it back into electricity using just one set of catalysts.
PCFCs consist of two electrodes separated by a membrane that allows protons across. At the first electrode, known as the air electrode, steam and electricity are fed into a ceramic catalyst, which splits the steam’s water molecules into positively charged hydrogen ions (protons), electrons, and oxygen molecules. The electrons travel through an external wire to the second electrode—the fuel electrode—where they meet up with the protons that crossed through the membrane. There, a nickel-based catalyst stitches them together to make hydrogen gas (H2). In previous PCFCs, the nickel catalysts performed well, but the ceramic catalysts were inefficient, using less than 70% of the electricity to split the water molecules. Much of the energy was lost as heat.
Now, two research teams have made key strides in improving this efficiency, and a new fuel cell concept brings biological design ideas into the mix. They both focused on making improvements to the air electrode, because the nickel-based fuel electrode did a good enough job. In January, researchers led by chemist Sossina Haile at Northwestern University in Evanston, Illinois, reported in Energy & Environmental Science that they came up with a fuel electrode made from a ceramic alloy containing six elements that harnessed 76% of its electricity to split water molecules. And in today’s issue of Nature Energy, Ryan O’Hayre, a chemist at the Colorado School of Mines in Golden, reports that his team has done one better. Their ceramic alloy electrode, made up of five elements, harnesses as much as 98% of the energy it’s fed to split water.
When both teams run their setups in reverse, the fuel electrode splits H2 molecules into protons and electrons. The electrons travel through an external wire to the air electrode—providing electricity to power devices. When they reach the electrode, they combine with oxygen from the air and protons that crossed back over the membrane to produce water.
The O’Hayre group’s latest work is “impressive,” Haile says. “The electricity you are putting in is making H2 and not heating up your system. They did a really good job with that.” Still, she cautions, both her new device and the one from the O’Hayre lab are small laboratory demonstrations. For the technology to have a societal impact, researchers will need to scale up the button-size devices, a process that typically reduces performance. If engineers can make that happen, the cost of storing renewable energy could drop precipitously, thereby moving us closer to cheap abundant electricity at scale, helping utilities do away with their dependence on fossil fuels.